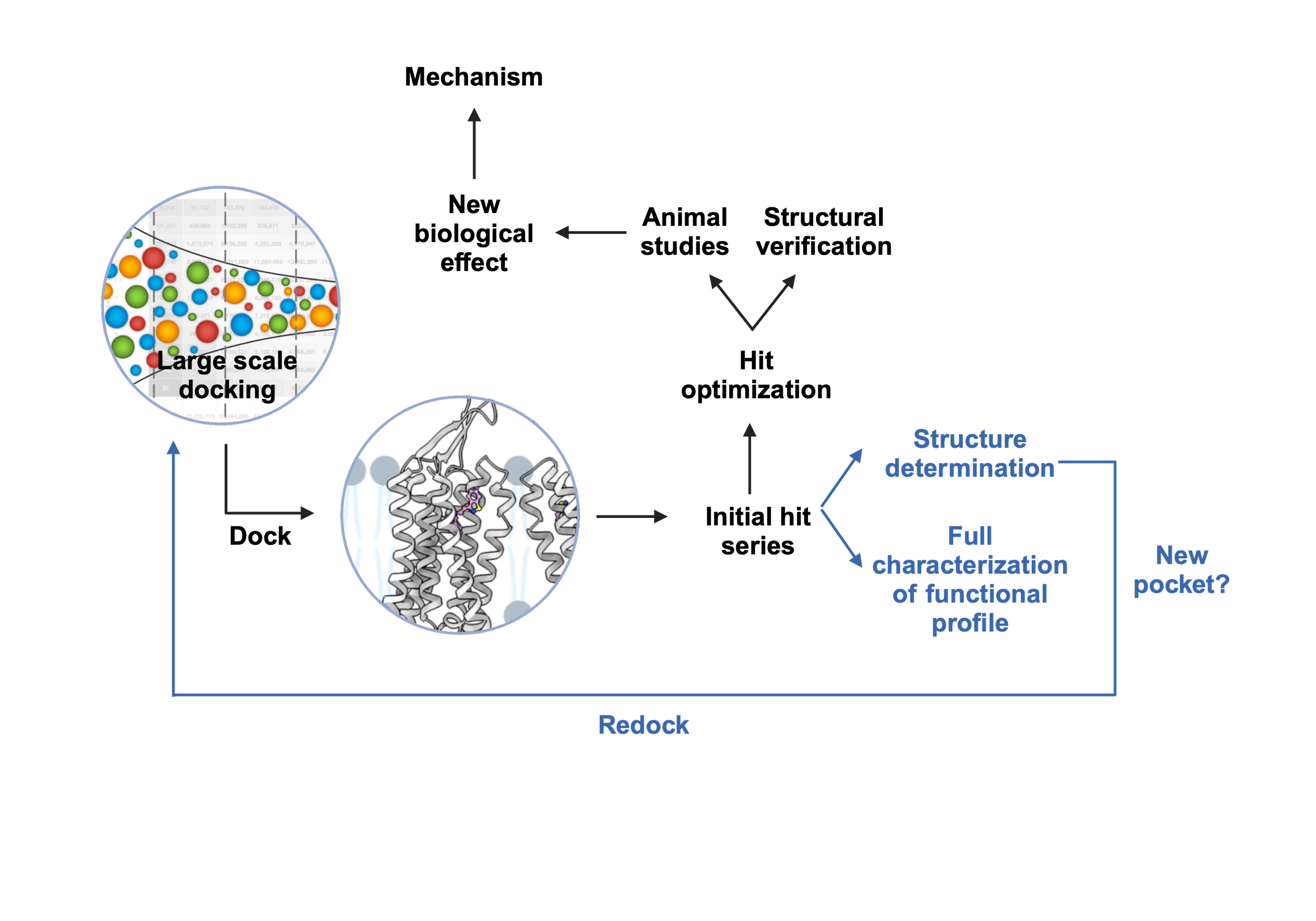
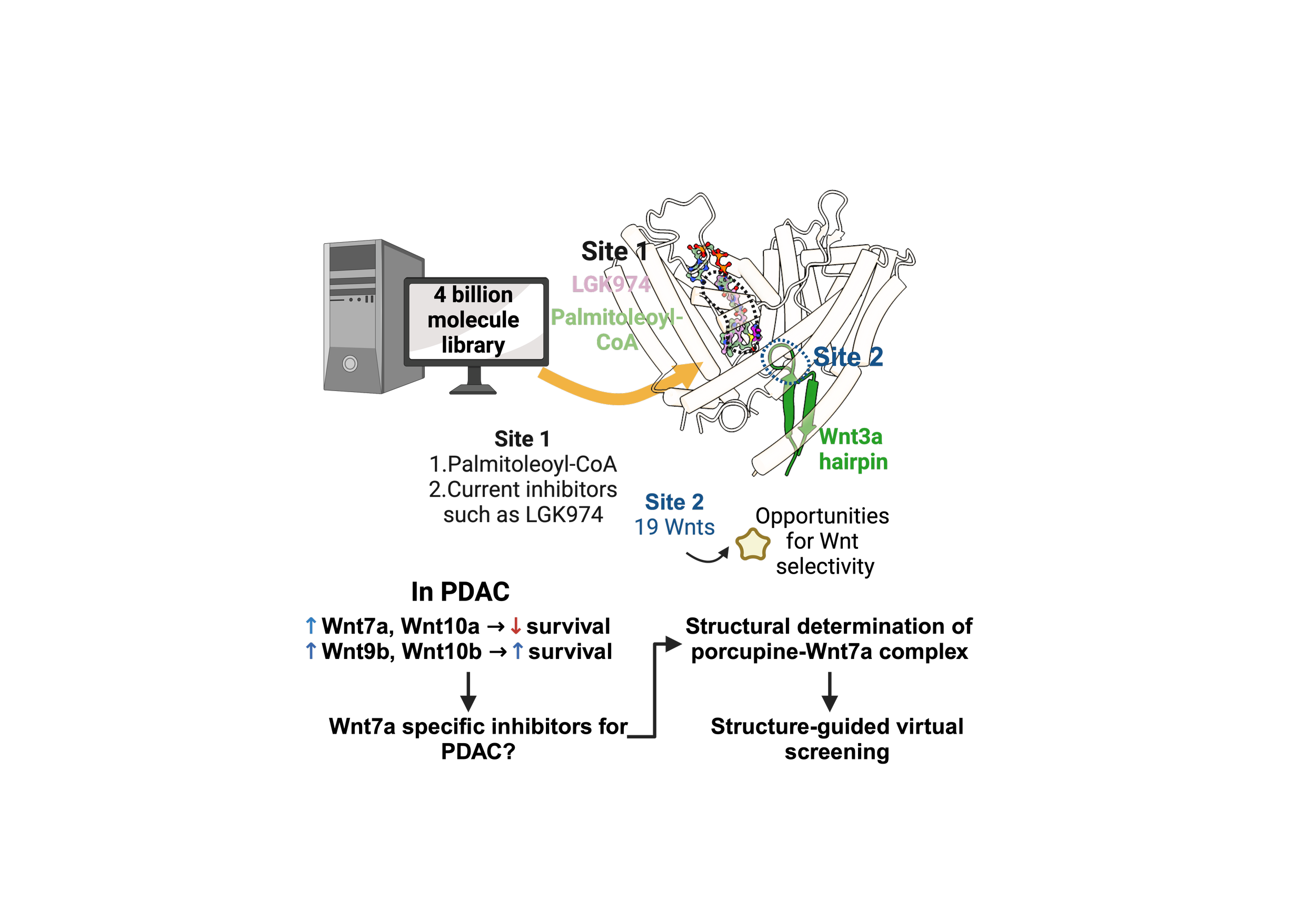
Pancreatic ductal adenocarcinoma (PDAC) has a 5-yr survival rate of ~13%, a high relapse rate of 80% and is resistant to traditional therapies. Over 90% of PDAC cases feature mutations in the KRAS gene, which not only promotes cell proliferation but also increases reactive oxygen species, making these cancer cells vulnerable to a cell death process called ferroptosis. PDAC is further characterized by abnormal activation of embryonic signaling pathways such as Hedgehog, Notch, and Wnt, which are usually inactive in an adult pancreas. Wnt signaling, in particular, is crucial in PDAC progression, metastasis, and chemoresistance.
Porcupine catalyzes the transfer of a palmitoleoyl-CoA to all 19 Wnts in human to initiate Wnt signaling. The binding site for current porcupine inhibitors (Site 1) overlaps with the palmitoleoyl-CoA site, inhibiting porcupine unbiasedly. Non-biased inhibition of porcupine is highly desirable for better efficacy and lower toxicity, which maybe achieved through selective inhibition of porcupine through Site 2.
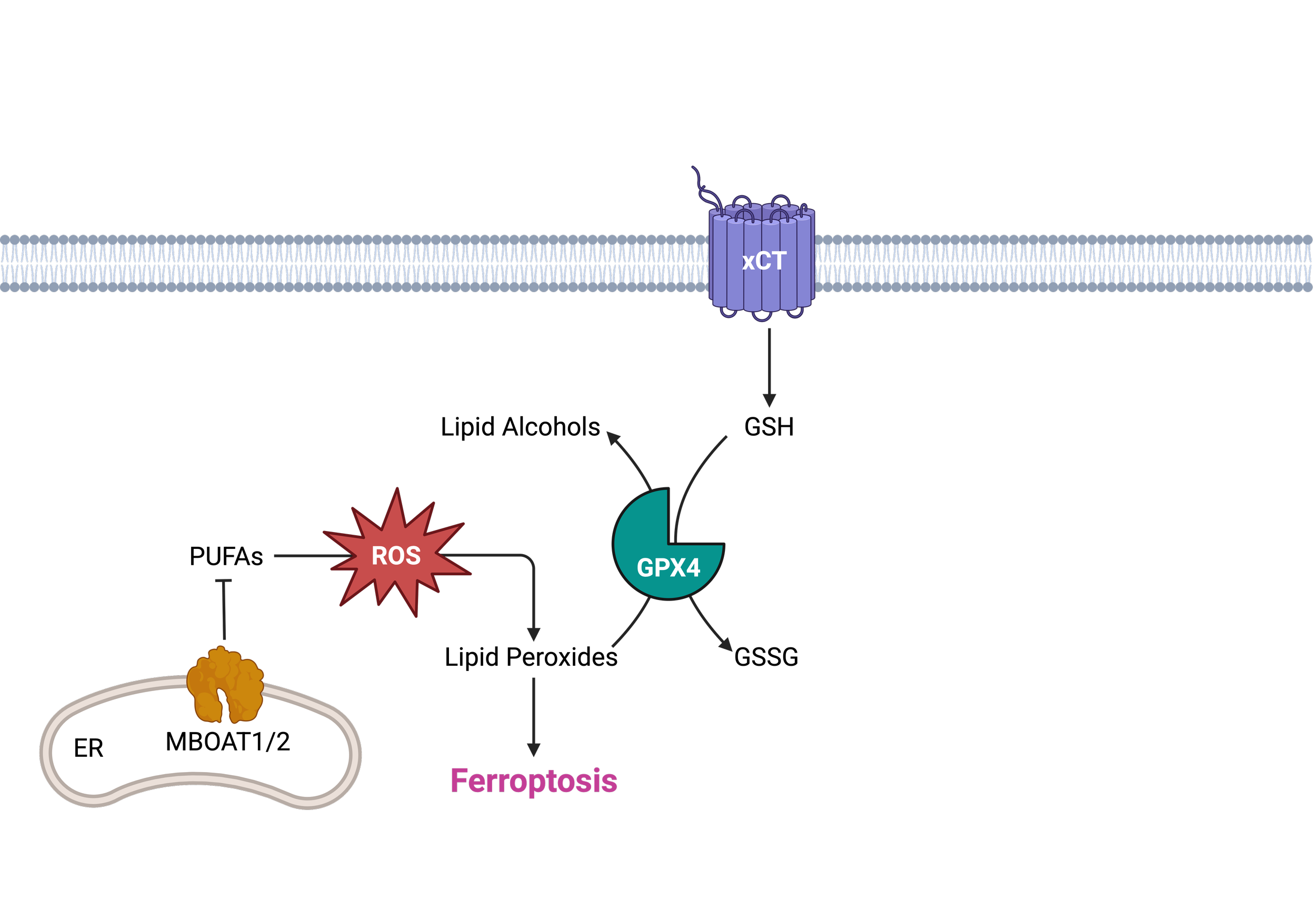
Ferroptosis is a cell death process driven by iron-dependent phospholipid peroxidation and is a potential therapeutic approach to selectively eliminate cancer cells. A recent CRISPR screen identified MBOAT1 and MBOAT2 as ferroptosis suppressors, regulated by estrogen receptor (ER) or androgen receptor (AR) signaling. In pancreatic cancer, MBOAT1 overexpression is an unfavorable biomarker for survival, while MBOAT2 overexpression is correlated with KRAS activation and reduced CD8+ T-cell infiltration. Given their clinical relevance, developing pharmacological tools to investigate MBOAT1 and MBOAT2 functions and their clinical applications is highly desirable.